[ad_1]
In 1947, Jacques Monod, a biochemist on the Pasteur Institute, observed that Escherichia coli metabolized lactose offered that glucose, the micro organism’s preferred sugar provide, was absent.1 He began discovering out the system by investigating the lactose-digesting enzyme, β-galactosidase.2 Although he acknowledged assorted substrates of the enzyme, the regulatory mechanism that managed the metabolic swap remained elusive.3
When biologist François Jacob joined the institute in 1950, his evaluation on how lambda bacteriophages built-in their genome into E. coli couldn’t seem additional unrelated to Monod’s enzyme analysis.4 Jacob used conjugation, the swap of nucleic acid between micro organism by a bridge-like connection, to evaluate how phage-infected E. coli developed resistance to secondary phage infections; Jacob and others interrupted this course of to analysis the swap of the phage genetic supplies and order of genes.5
It offers a technique to leap off and say, ‘how can we use this data to develop new, modern switches that may be utilized in eukaryotic strategies and mammalian strategies.’
—Mitchell Lewis, School of Pennsylvania
Jacob and Monod realized that they could use an equivalent methodology to change genes between common micro organism and mutants that each always expressed or absolutely lacked β-galactosidase. Based mostly totally on these experiments, the two scientists demonstrated in 1959 that an inhibitory gene product managed the expression of β-galactosidase.6 This inhibition was inducible, guaranteeing that the micro organism solely produced the lactose-digesting enzyme when lactose was on the market to digest. Whereas scientists had beforehand understood that genes contained the instructions for establishing all of the cell’s necessary proteins, this provided one among many first examples of gene regulation by repression in response to changes inside the setting.7
Jacob drew a parallel between the findings of β-galactosidase induction and bacteriophage manufacturing; every gave the look to be managed by some regulatory mechanism that repressed expression of the genes. Using lactose metabolism mutants, Jacob and Monod determined that the repressing issue sure to a space of DNA, which they often called the operator, and that this managed the expression of a cluster of genes, which they often called the lac operon, that was accountable for lactose digestion.8
“Earlier to that work, the working model had been that the β-galactosidase, the enzyme produced by the lac operon, was there regularly,” acknowledged Stephen Busby, a biologist on the School of Birmingham. “(Scientists thought) it was made there regularly inside the cell, nonetheless its train was regulated.” Jacob and Monod’s discovery of a genetic mechanism of β-galactosidase expression would set the stage for unraveling the mechanisms of gene regulation.
The lac Operon Outlined
In 1964, Jacob and Monod acknowledged the promoter space for RNA polymerase.9 Only a few years later, one different workers mapped the genes of the lac operon—the repressor, promoter, operon, β-galactosidase, and two additional structural genes, lactose permease and a transacetylase.10
Whereas Jacob, Monod, and others beforehand confirmed that lactose metabolites containing galactose residues activated the lac operon, the true inducer remained elusive.11–13 Then, in 1972, a workers acknowledged allolactose, a lactose metabolite, as a result of the lac operon inducer.14 Throughout the presence of lactose, residual β-galactosidase produces allolactose, which binds to the repressor to launch it from the DNA. The lac operon genes are expressed to digest the on the market lactose until this sugar is depleted, at which degree the operon shuts down.
Nonetheless, the lac operon was faraway from solved. “(It) turned out that there was a class of gene regulatory proteins that weren’t repressors, (instead,) that they had been activators,” Busby acknowledged. Low cellular glucose leads to the manufacturing of cyclic adenosine monophosphate (cAMP); this molecule in flip binds to the cAMP receptor protein (CRP). This sophisticated binds the lac operon, stabilizing the RNA polymerase to improve transcription of the lac operon genes when the repressor lifts.15,16
One different shock obtained right here when scientists discovered that the operon had two promoters.17 Every drove lac operon gene expression if lactose was present, nonetheless one was specialised for induction beneath low glucose circumstances. When CRP binds to stabilize the RNA polymerase, it moreover blocks one amongst these promoters; the on the market sequence is a stronger promoter, rising gene expression and the manufacturing of lactose-digesting gear in low-energy circumstances compared with that from the second promoter.18–20
“That always struck me as really, really thrilling to know the magic finger that someway can choose one gene and say, ‘Gee, you will be turned on now,’” Busby acknowledged, who studied the place of cAMP/CRP inside the recruitment of RNA polymerase inside the lactose and totally different operons.
Walter Gilbert and Benno Müller-Hill answered one different question regarding the lac operon after they isolated a protein, later named LacI, that was accountable for repressing the system, confirming earlier indications that the repressor was a protein.21,22 Later, Gilbert and his colleague Allan Maxam confirmed that LacI was a tetrameric protein with 4 potential binding web sites; they confirmed that two doable sure the operator sequencing, nonetheless the target behind the second set of subunits remained unclear.23

The Lac repressor can bind two operator sequences inside the lac operon to inhibit the transcription of those genes by forming a DNA loop.
Mitchell Lewis
In 1977, Müller-Hill’s group found two additional operator sequences inside the lac space.24 “There was this thriller: Why on this planet are there additional binding web sites for (LacI)?” acknowledged Jim Maher, a strategies biologist on the Mayo Clinic School of Medication and Science. He outlined that almost all textbooks illustrate the lac operon as a linear assemble of genes the place LacI binds to the DNA to cease gene expression by bodily blocking the entry of RNA polymerase. “That story is an oversimplification.”
Just about a decade after the invention of the additional operator sequences, scientists proposed that the carry out of these additional areas was to increase lac repression by DNA looping.25,26 The repressor could bind on to the primary operator by colliding with it, or, using the second set of DNA-binding subunits, LacI could bind to a distant web site first and be delivered to the first operator by this DNA construction and bind this sequence as correctly. “You make a rather a lot tighter swap if there’s quite a few sources of this protein that could be part of there, and you don’t want as rather a lot protein floating spherical to get the swap to go on and off,” Maher outlined.
The Regulation of the lac Operon The genes and obligatory sequences for the regulation of the lac operon are organized such that quite a few mediators can efficient tune expression of the three genes inside the operon itself—lacZ, lacY, and lacA. The gene lacI, which encodes the lac repressor, LacI, sits exterior the operon and is constitutively expressed. A cyclic adenosine monophosphate (cAMP) receptor protein (CRP) binding web site (C) sits in entrance of the promoter space the place there are two potential RNA polymerase binding web sites (P1 and P2). CRP controls the binding of RNA polymerase between P1 and P2 by binding C. Lastly, three operator areas, O1, O2, and O3, are spaced by the DNA space to modulate LacI binding and complete operon repression. Leaky Repression When glucose, nonetheless not lactose, is obtainable inside the cell, the LacI binds to the O1 sequence, stopping RNA polymerase from binding to the promoter space. Nonetheless, attributable to protein kinetics, a small amount of the lac operon genes could also be expressed if the polymerase binds when one repressor releases the DNA sooner than one different binds. Strong Activation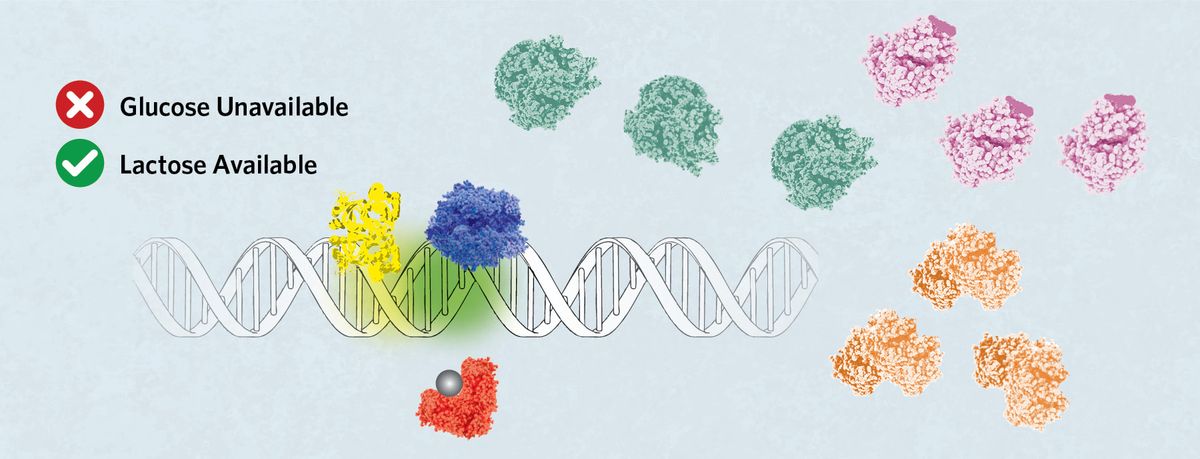 If glucose turns into unavailable nonetheless lactose is present, then allolactose (grey sphere) binds LacI, releasing it from the operator. cAMP, produced inside the absence of glucose, attaches to CRP, prompting its binding to the C web site. This directs the RNA polymerase to take a seat down on the P1 web site, which promotes robust expression of the lac operon to facilitate lactose digestion. Tight Repression modified from © istock.com, VanReeel; stock.adobe.com, molekuul.be; designed by erin lemieux
The repression of the lac operon inside the absence of lactose could also be improved by DNA looping, by way of which LacI binds to O1 and a second operator sequence, each O2 or O3. This may enhance the native focus of LacI, lowering transient expression that occurs when solely free-binding lac repressor is obtainable. Weak Activation When glucose and lactose are every on the market, allolactose releases LacI from the operator, allowing binding of the RNA polymerase. Nonetheless, inside the absence of cAMP to permit CRP binding, the polymerase binds each P1 or P2 and does not keep on the DNA as efficiently, leading to low expression of the lac operon genes. |
Resolving the lac Repressor Building: Regulation is Loopy
Throughout the early Nineties, 30 years after Jacob and Monod’s discovery, the 3D building of the lac repressor remained elusive. Mitchell Lewis, a structural biologist on the School of Pennsylvania, was a postdoctoral fellow at Harvard School beneath Mark Ptashne, a molecular biologist who acknowledged the sequence and building of the lambda bacteriophage repressor.27,28
At a celebration for this feat, Lewis instructed Gilbert that he meant to unravel the development of LacI. “He checked out me and acknowledged, ‘Mitch, higher males than you may need tried,’” Lewis recalled.
On the time, producing and purifying sufficient protein for X-ray crystallography was troublesome, nonetheless advances in high-performance liquid chromatography improved the yield. Lewis and his workers solved the development of the tetramer alone, sure to the operator sequence, and sure to an allolactose analog.29 “That was basically probably the most thrilling issue,” Lewis acknowledged. “You would presumably actually see how this molecule switched from completely totally different conformations to each bind DNA or launch the DNA.”
Subsequently, scientists solved crystal constructions that clarify the intricacies of DNA looping in lac repression.30,31 Nonetheless, these intense investigations into the inside workings of a bacterial gene group do not exist in isolation. “The most important issue was that, after (the invention of a gene regulatory protein), of us discovered that these transcription elements regulated gazillions of points,” Busby acknowledged.
“It’s additional than merely bacterial genetics,” Lewis acknowledged regarding the significance of the lac operon. “It actually works in a barely completely totally different method in eukaryotic strategies, nonetheless the first idea is you wish to have the flexibility to flip genes on and off.”
For example, the expression of the gene sex-determining space Y (Sry) in numerous mammals prompts a program involving quite a few totally different genes to promote the occasion of male traits inside the species.32 Furthermore, understanding the transcriptional program in enchancment acknowledged the so-called Yamanaka elements that gave rise to induced pluripotent stem cells.33
Turning the Inducible lac Operon proper right into a Instrument
As quickly as researchers established most of the inside workings of the lac operon, they began to utilize this method to their profit. “Molecular biologists love devices,” Maher acknowledged. “When we’ve got to flip genes on and off, which we recurrently do in molecular biology, one among many first strategies we do it is to indicate to the lac operon.”
Using a lactose analog or altering the lactose administration with tetracycline, scientists use the principles of the lac operon to selectively administration expression of genes of curiosity.34 Researchers even utilized bacterial genetics on to mammalian genetics, using the lac operon as a swap to control expression in a gene swap experiment in mice.35

Jim Maher and his collaborators developed an artificial kinking protein that facilitated DNA looping with the Lac repressor.
Wilma Olson
Many researchers are moreover making use of knowledge from the lac operon to tangential strategies and questions. For example, Maher makes use of the DNA construction of the lac operon to know the way DNA makes switches and wraps spherical accent proteins to handle gene expression or for normal packaging. “We exploit that system, not because of we’re so concerned to find out lactose biology, nonetheless we’re very inside the stiffness of this piece of intervening DNA,” he acknowledged. They’re moreover concerned in making use of micro loops like these inside the lac operon to eukaryotic strategies to repress genes.
For the time being, the lac operon stays a mainstay in biology lectures as a result of the paradigm for gene regulation. The regulatory system and its derivatives are typically used as a leaping off degree for industrial and therapeutic protein manufacturing, and scientists proceed to improve upon these fashions.36–38 “It offers a technique to leap off and say, ‘how can we use this data to develop new, modern switches that may be utilized in eukaryotic strategies and mammalian strategies?’” Lewis acknowledged.
- Monod J, Audureau A. Mutation and enzymatic adaptation in Escherichia coli-mutabile. Ann Inst Pasteur. 1946;72(11/12):868-878.
- Monod J, et al. Sur la biosynthese de la β-galactosidase (lactase) chez Escherichia coli. La specificite de l’induction. Acta Biochim Biophys. 1951;7:585-599.
- Monod J, et al. La cinétique de la biosynthèse de la β-galactosidase chez E. coli considérée comme fonction de la croissance. Acta Biochim Biophys. 1959;9:648-660.
- Jacob F. Transduction of lysogeny in Escherichia coli. Virology. 1955;1(2):207-220.
- Kaiser AD, Jacob F. Recombination between related temperate bacteriophages and the genetic administration of immunity and prophage localization. Virology. 1957;4(3):509-521.
- Pardee A, et al. The genetic administration and cytoplasmic expression of “Inducibility” inside the synthesis of β-galactosidase by E. coli. J Mol Biol. 1959;1(2):165-178.
- Jacob F, Monod J. Genes determining the development and regulatory genes inside the biosynthesis of proteins. C R Acad Sci. 1959;249:1282-1284.
- Jacob F, et al. The operon: a gaggle of genes with expression coordinated by an operator. C R Acad Sci. 1960;250:1727-1729.
- Jacob F, et al. The promoter, a genetic issue necessary for the expression of an operon. C R Acad Sci. 1964;258:3125-3128.
- Miller JH, et al. The promoter-operator space of the Lac operon of Escherichia coli. 1968;38(3):413-420.
- Burstein C, et al. Operate of lactose and its metabolic merchandise inside the induction of the lactose operon in Escherichia coli. Biochim Biophys Acta. 1965;95(4):634-639.
- Nakada D, Magasanik B. The roles of inducer and catabolite repressor inside the synthesis of β-galactosidase by Escherichia coli. J Mol Biol. 1964;8(1):105-127.
- Jacob F, Monod J. Genetic regulatory mechanism inside the synthesis of proteins. J Mol Biol. 1961;3(3):318-356.
- Jobe A, Bourgeois S. lac repressor-operator interaction: VI. The pure inducer of the lac operon. J Mol Biol. 1972;69(3):397-404.
- Pastan I, Perlman R. Cyclic adenosine monophosphate in micro organism: In numerous micro organism the synthesis of inducible enzyme requires this cyclic nucleotide. Science. 1970;169(3943):339-344.
- Maquat LE, Reznikoff WS. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978;125(4):467-490.
- Beckwith J, et al. Proof for two web sites inside the lac promoter space. J Mol Biol. 1972;69(1):155-160.
- Malan TP, McClure WR. Twin promoter administration of the escherichia coli lactose operon. Cell. 1984;39(1):173-180.
- Straney DC, et al. Synergy between Escherichia coli CAP protein and RNA polymerase inside the lac promoter open sophisticated. J Mol Biol. 1989;206(1):41-57.
- Malan TP, et al. Mechanism of CRP-cAMP activation of lac operon transcription initiation activation of the P1 promoter. J Mol Biol. 1984;180(4):881-909.
- Gilbert W, Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci USA. 1966;56(6):1891-1898.
- Müller-Hill B, et al. Specificity of the induction of the enzymes of the lac operon in Escherichia coli. J Mol Biol. 1964;10(2):303-318.
- Gilbert W, Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci USA. 1973;70(12):3518-3584.
- Kania J, Müller-Hill B. Growth, isolation and implications of repressor-galactosidase ·ß-galactosidase hybrid molecules. Eur J Biochem. 1977;79(2):381-386.
- Oehler S, et al. The three operators of the lac operon cooperate in repression. EMBO. 1990;9:973-979.
- Mossing MC, Doc MT. Upstream operators enhance repression of the lac promoter. Science. 1986;233(4766):889-892.
- Hochschild A, et al. Repressor building and the mechanism of constructive administration. Cell. 1983;32(2):319-325.
- Anderson JE, et al. Building of the represser–operator sophisticated of bacteriophage 434. Nature. 1987;326(6116):846-852.
- Tempo HC, et al. lac repressor: Crystallization of intact tetramer and its complexes with inducer and operator DNA. Proc Natl Acad Sci USA. 1990;87(5):1870-1873.
- Lewis M, et al. Crystal building of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271(5353):1247-1254.
- Friedman AM, et al. Crystal building of the lac repressor core tetramer and its implications for DNA looping. Science. 1995;268(5218):1721-1727.
- Larney C, et al. Switching on intercourse: Transcriptional regulation of the testis-determining gene Sry. Enchancment. 2014;141(11):2195-2205.
- Takahashi Okay, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and grownup fibroblast cultures by outlined elements. Cell. 2006;126(4):663-676.
- Skerra A. Use of the tetracycline promoter for the tightly regulated manufacturing of a murine antibody fragment in Escherichia coli. Gene. 1994;151(1-2):131-135.
- Hu MC-T, Davidson N. The inducible Iac operator-repressor system is purposeful in mammalian cells. Cell. 1987;48(4):555-566.
- Maccormick CA, et al. Growth of a food-grade host/vector system for Lactococcus lactis primarily based totally on the lactose operon. FEMS Microbiol Lett. 1995;127(1-2):105-109.
- Graumann Okay, Premstaller A. Manufacturing of recombinant therapeutic proteins in microbial strategies. Biotechnol J. 2006;1(2):164-186.
- Lalwani MA, et al. Optogenetic administration of the lac operon for bacterial chemical and protein manufacturing. Nat Chem Biol. 2021;17(1):71-79.
[ad_2]
Provide hyperlink